

This theory was adopted by Niels Bohr in 1913 who theorised that electrons could orbit the nucleus in a circular orbits and that the distance of the electron to the nucleus was fixed unless it moved between energy levels with the absorption or emission of light. Max Planck and Albert Einstein in the field of physics postulated that light energy can be absorbed and emitted as quanta. Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate would make a sharp-edged picture, while alpha particles beamed through a sheet of mica only 20 micrometres (or about 0.002 cm) thick would make an impression with blurry edges. But what does that statement mean Geographical discovery usually means that one sees a place for the first time. Rutherford overturned Thomson’s model in 1911 with his famous gold-foil experiment, in which he demonstrated that the atom has a tiny, massive nucleus. We read this in textbooks and in popular writings. It was not until the earlier 20th Century that the scientific community arrived at the modern day atomic model. Rutherford at Manchester, 19071919 Ernest Rutherford discovered the nucleus of the atom in 1911. Rutherfords gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Now the atomic model had a central particle and electrons around it, reversing he plum pudding model of Thomson. He named this new fundamental particle as a proton. Description of his model: Rutherford proposed that atoms consisted of a small dense center filled with positive charges.
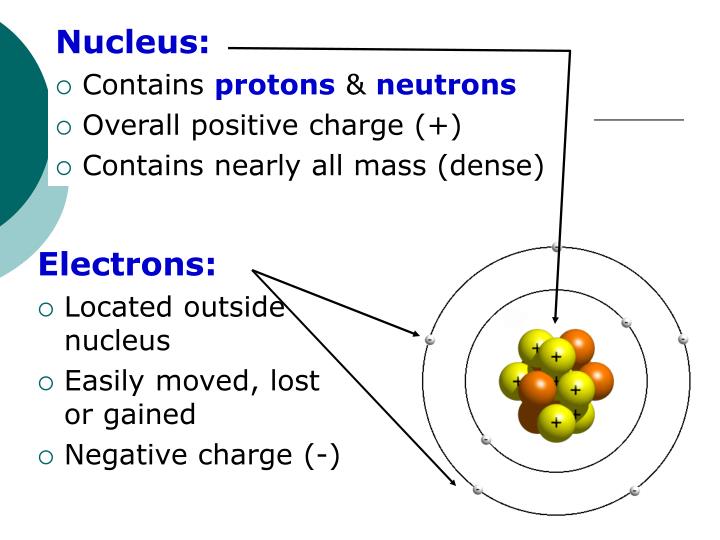
Rutherford conducted a number of experiments with hydrogen nuclei and nitrogen in air using alpha particles and after a number of theories concluded that the hydrogen atom made up other atoms. Rutherford used a-ray bullets to distinguish between the plum-pudding. First modern concept of atomic structure all of the positive charge and most of the mass of the atom are contained in a compact. Rutherford further followed this up in 1917 when he proved that a hydrogen nucleus (1 proton) is present in other nuclei of different elements most notably nitrogen gas in the air.


 0 kommentar(er)
0 kommentar(er)
